NEONATAL HSV INFECTION
This chapter was updated in October 2025, in line with international guidelines.
| KEY POINTS |
|---|
| Neonatal herpes simplex virus (HSV) infection is a rare, but potentially fatal, disease of babies, that occurs within the first 4–6 weeks of life. Symptoms are nonspecific and a high index of suspicion is required. All infants <4 weeks undergoing blood culture should have HSV PCR included. |
| Most neonatal HSV infections are acquired via the following transmission sources: 70-85% perinatal, 10-25% postnatal (caregivers/relatives), ~5% antenatal, generally all from an unrecognised HSV infection. |
| Any neonate that develops skin vesicles or atypical bullous, pustular skin lesions, especially on the scalp or face (vaginal deliveries) or over the buttocks (breech presentation), must be referred to a paediatrician/infectious diseases specialist immediately. |
| Specialist obstetric and paediatric advice on management and anticipatory guidance for neonatal HSV infection should be sought for a pregnant person with a history of genital herpes and active lesions at term, especially in the high-risk situation where a first episode occurs within 6 weeks of delivery. |
Neonatal herpes is a rare but potentially serious infection that may be associated with significant morbidity and mortality. Rates of neonatal HSV infection vary between countries, being lower in Western Europe (1.15 and 3.2 cases per 100,000 live births in France and the Netherlands, respectively)(1-2), intermediate in Scandinavia (6.5 cases per 100,000 live births in Sweden),(3) Canada (5.9 cases per 100,000 live births),(4) and the United Kingdom (6.9 cases per 100,000 live births),(5) and higher in the USA (9.6 cases per 100,000 live births).(6) There can also be marked differences in local incidence rates within countries.(7) The reasons underlying these differences in reported neonatal HSV infection rates are probably multifactorial, including variations in case definition and study design, and differences in maternal HSV acquisition rates. Reliable New Zealand data is lacking, but a prospective national active surveillance study conducted in Australia from 1997 to 2011 reported an incidence of 3.7 per 100,000 live births.(8, 9) The incidence of neonatal HSV infection in Australia was stable over this time period but the number of HSV-1 infections was higher than that of HSV-2 infections. In addition, the mortality rate from neonatal HSV infection decreased in the later part of the evaluation period. Prospective longitudinal data such as these are helpful in providing accurate incidence and epidemiological data to guide effective education and prevention strategies.(10)
Transmission from the mother to the foetus or neonate
Neonatal HSV infection may be acquired antenatally, at the time of delivery or postpartum.(7) About 70-85% of neonatal HSV infections are acquired during labour (perinatally) through direct contact with infected genital secretions, <5% are acquired in utero, and 10-25% are acquired postpartum.(5) Of infants with proven HSV infection, 80% have no documented history of herpes infection in either the pregnant person or their partner. The decreasing prevalence of HSV-1 in childhood increases the susceptibility of young adults to genital HSV-1, and hence increases the risk of neonatal HSV infection.(11)
Although reactivation of HSV-1 is less common than that of HSV-2, there is evidence that reactivated HSV-1 may be more readily transmitted to the neonate. Maternal infection classification - primary, non-primary first episode, recurrent - with associated transmission risks (50%, 25%, <1%). The same strategies are required for prevention of both HSV-1 and HSV-2.(12) Maternal antibodies cross the placenta and are protective. If lesions are present at delivery, the risk of transmission is small (0.25–3%).(13) Preterm infants are at higher risk due to reduced maternal antibody transfer. The risk for transmission of reactivated HSV-2 infection appears to be less than 1%.(7) Pregnant people infected with both HIV and HSV-2 are at greater risk of transmitting HSV-2 because HSV-2 shedding is increased in HIV co-infected people.(14)
Maternal risk groups
The risk of neonatal HSV infection depends primarily on the type and timing of maternal infection. Transmission risk is highest when maternal infection is acquired late in pregnancy, before protective antibodies have developed, and lowest when infection is recurrent.
Risk stratification
Highest risk
- First episode of genital HSV (primary or non-primary) within 6 weeks of delivery, especially if delivered vaginally or after rupture of membranes >4 hours.
- Estimated vertical transmission risk up to 50% with primary first infection.
- Management: treat the neonate as suspected HSV infection - full investigations and immediate IV aciclovir.
High risk
- Vaginal birth to a pregnant person with active genital HSV disease and no prior history of HSV.
- Elective Caesarean section in a pregnant person with first episode genital herpes within 6 weeks of delivery.
- Estimated transmission risk ~25% with non-primary first infection.
- Management: collect full diagnostic specimens, consider empiric IV aciclovir depending on clinical status.
Low risk
- Maternal recurrent genital herpes at the time of delivery.
- First episode genital HSV earlier in pregnancy (>6 weeks before delivery).
- Transmission risk ~0.25-3% if lesions present, <1% if asymptomatic.
- Management: surveillance (PCR swabs at 24-48 hours), parental education, no empiric aciclovir if infant well.
Lowest risk
- History of recurrent genital HSV during pregnancy but no lesions or prodrome at delivery.
- Transmission risk <0.05%.
- Management: routine neonatal care, parental education regarding symptoms, no special investigations if infant well.
Intrauterine infection
Intrauterine infection results from either transplacental HSV transmission or an ascending HSV infection from the cervix. Rarely, primary maternal infection can result in disseminated infection of the foetus, presenting as skin lesions, chorioretinitis or microcephaly or hydrocephalus at birth.(15) A minority of infants with late intrauterine HSV infection will present at delivery with skin or eye lesions. The long-term outlook for infected infants is poor, but the likelihood of antiviral therapy being successful in those with late infection is greater than in those with more long-standing intrauterine
infection.(16)
Perinatal infection
The highest risk of HSV transmission from a mother to a neonate is at delivery; contact with HSV-infected secretions in the birth canal accounts for the large majority of neonatal HSV infections.(7) The site of entry is usually the eye, nasopharynx or an abrasion secondary to scalp electrodes or forceps. About 60–80% of infants with neonatal HSV disease are born to people with unrecognised infection.(17, 18)
Several factors influence the risk of a newborn acquiring HSV infection, the most important of which is whether the mother has newly acquired or recurrent genital disease.(13, 19) The risk is greatest when a previously seronegative person acquires genital herpes (HSV-1 or HSV-2) at or near the time of delivery. In this case, the risk of neonatal HSV infection is 50%, while vertical transmission rates of 25% are found in those with a non-primary first episode (i.e. infection with one type of HSV [e.g. HSV-2] in the presence of antibodies to the other type of HSV [e.g. HSV-1]).
Transmission rates are lowest for pregnant people who acquire herpes before pregnancy, with a risk of about 0.05% for these people if they have no signs or symptoms of an outbreak at delivery.(19, 20) If lesions are present at delivery, the risk of transmission is small, but still significant (0.25–3%).(13) High maternal titres of type-specific neutralising antibody are associated with a substantially lower risk and severity of neonatal infection, while risk factors for neonatal infection include the use of invasive obstetric procedures (e.g. foetal scalp electrodes), method of delivery, and prolonged rupture of membranes.(13) Data from Australia and Canada indicate that an increasing proportion of genital and neonatal herpes infection are due to infection with HSV-1 strains.(4, 8)
Postnatal infection
Sources of postnatal HSV infection include maternal skin and oral lesions, and HSV lesions on caregivers, other family members and medical staff that have close contact with the newborn. Breast milk transmission has not been reported, but neonatal disease after contact with maternal breast herpes lesions has been reported.
Disease classification
Intrauterine HSV infection
This is rare and usually occurs after primary herpes infection in pregnancy. Overall, the long-term outlook for infants with intrauterine HSV infection is poor. Transplacental transmission before 20 weeks of gestation may cause spontaneous abortion (miscarriage) in as many as 25% of cases.(21) In contrast to neonatal herpes infection, signs of intrauterine HSV infection are present at delivery and may include intrauterine growth retardation, hydranencephaly, chorioretinitis and skin scarring. A minority of neonates with intrauterine HSV infection will present at delivery with skin or eye lesions. There is often a history of prolonged rupture of membranes, for as long as 2 weeks, and in these cases the likelihood of antiviral therapy being successful is higher than that for newborns with more long-standing intrauterine infection and complications such as hydranencephaly, but a small group will have severe, disseminated disease or fatal pneumonitis.(16)
Neonatal HSV infection
There is no clear pattern of signs and symptoms that identifies babies with neonatal HSV disease, meaning that a high index of suspicion is required. Presenting symptoms include fever, lethargy, seizures and respiratory distress, although fever may be absent initially.(18) Only 40% of affected infants will have vesicles at presentation and some will have no vesicles at any time during the course of their illness.(22, 23) The mortality rate is highest in neonates with altered consciousness, seizures, disseminated intravascular coagulation, and prematurity.(16, 24)
The usual age of symptom onset in neonatal HSV infection is between 5 and 21 days, but there may be a delay in diagnosis if the significance of the symptoms is not initially recognised. Physicians caring for sick infants in the first 6 weeks of life should always be aware that neonatal HSV infection remains a possibility, even when no parental history of HSV infection is given.(4)
Classification and frequency of neonatal HSV infection symptoms are shown in Table 2 and described in more detail in the text below. There is often overlap between these categories and patients can switch from one category to another if not treated early.
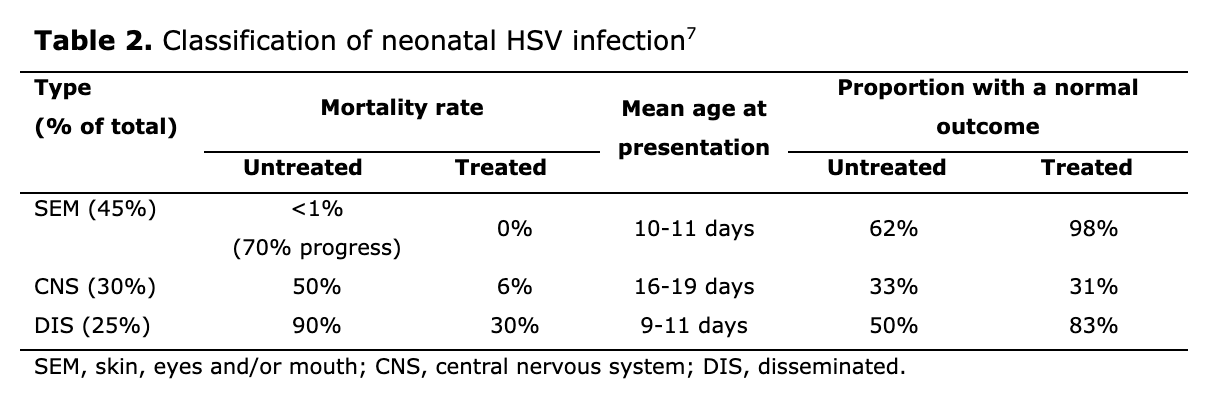
Skin, eyes and/or mouth (SEM) infection(18)
Nearly half of all neonates with HSV infection will present with lesions that are confined to the skin, eyes or mucous membranes. This is the most easily recognised form of the disease, with most babies having vesicular skin lesions at sites of trauma, such as over the presenting body part, foetal scalp electrode sites and eyelid margins. Lesions usually appear when the infant is 1–2 weeks of age but are sometimes evident shortly after birth when prolonged rupture of membranes has been present. Typically, vesicles overlie an erythematous base and contain clear or slightly cloudy fluid. Eye involvement needs to be evaluated carefully because this can initially be asymptomatic, and early ophthalmologic review is needed if symptoms appear.
Although rarely fatal if lesions are confined to skin and mucosal sites, many neonates progress to either the disseminated or CNS forms of the disease if they do not receive antiviral treatment. In addition, more than one-third of those with untreated localised SEM lesions develop signs of major neurological impairment such as microcephaly, spastic quadriplegia or sensory loss by 12 months of age. A study of infants with presumed SEM disease reported that 24% had HSV DNA detected in their cerebrospinal fluid based on CSF by polymerase chain reaction (PCR) testing, suggesting that HSV can infect the CNS without causing overt neurological symptoms.(25)
There are data to suggest that three or more recurrences of cutaneous vesicles in the first 6 months of life are predictive of poor neurological
outcome.(26) Specifically, the likelihood of normal development is nearly 100% when there are fewer than three recurrences within the first 6 months of life compared with only 79% when three or more recurrences occur during this period.(27) At the time of such episodes, PCR detection of HSV-DNA in the CSF may explain the emergence of new neurological deficits.(27)
Central nervous system (CNS) disease
Almost one-third of neonates with HSV infection will have only encephalitis. Affected infants usually present at between 10 days and 4 weeks of age and have symptoms of fever or temperature instability, lethargy, poor feeding and irritability, followed by seizures, a bulging fontanelle and focal neurological signs. CSF findings typically include a white blood cell count of 50–100 x106/L, a predominance of mononuclear cells, normal to low glucose, and elevated protein concentrations. Many neonates with CNS disease lack skin lesions at presentation, but 60–70% will have skin vesicles at some point during the disease course.(18)
The mortality rate of untreated CNS disease approaches 50%, with most survivors suffering severe neurological impairment. Morbidity is higher in infants with HSV-2 infection than in those with HSV-1 infection,(7) and there is little improvement in morbidity with the use of high-dose aciclovir. All neonatal CNS survivors should receive 6 months of oral suppressive aciclovir.
Disseminated disease (DIS)
Disseminated disease develops in about one-quarter of neonates with HSV infection. It is more common in preterm infants and has the worst prognosis. Symptoms generally develop in the first 14 days of life. Clinical presentation is similar to that of sepsis, with respiratory distress, haemodynamic instability, jaundice, hepatomegaly, elevated liver enzymes, bleeding with associated coagulopathy, and seizures with signs of meningitis, encephalitis or respiratory failure. Vesicular skin lesions may be absent in up to 50% of cases. Mortality in untreated patients is approximately 90%, and may still be as high as 20–30% even with antiviral therapy. All neonatal disseminated survivors should receive 6 months of oral suppressive aciclovir.
Differential diagnoses
The bacterial pathogens responsible for neonatal sepsis (which sometimes includes skin lesions that may be mistaken for disseminated or CNS HSV infection) include group B streptococcus, Listeria monocytogenes and gram-negative bacilli. Cutaneous infections resulting in vesicular lesions similar to neonatal HSV are bullous impetigo, varicella zoster, enteroviruses and disseminated CMV infection. Other infectious agents that might be considered are toxoplasmosis, rubella and syphilis. Finally, non-infectious cutaneous disorders that could be confused with neonatal HSV infection include erythema toxicum, neonatal pustular melanosis, acropustulosis and incontinentia pigmenti.
Management of neonatal HSV infection
Evaluation
The poor prognosis associated with untreated neonatal HSV infection means that early diagnosis is essential and therefore, a high level of suspicion is required. Valuable information could be provided by the lead maternity caregiver. Many cases of neonatal HSV infection present with a sepsis-like clinical picture but do not have any identifiable risk factors for sepsis. It is also important to remember that many infants with disseminated or CNS disease due to HSV infection will initially lack skin lesions to assist in a timely diagnosis, most lack a parental history of genital herpes.(7, 22, 23)
This means that physicians should consider neonatal HSV infection in infants younger than 6 weeks of age who have vesicular or atypical bullous, pustular skin lesions or a progressive febrile illness without a confirmed bacterial cause, which is unresponsive to antibiotics and associated with one or more of the following: skin vesicles, hepatomegaly, liver dysfunction, pneumonitis, thrombocytopenia, coagulopathy, or seizures. Other factors that may be of diagnostic importance in a neonate without a rash are maternal fever, respiratory distress requiring mechanical ventilation, and CSF
pleocytosis.(17)
There should be a daily check for the presence of skin and oral lesions, especially on the scalp and face (vaginal deliveries) or over the buttocks (breech presentation) because these may develop later in the course of disseminated and CNS disease. The index of suspicion is heightened by progressive liver function abnormalities, particularly during the first week of life. When neonatal HSV infection is considered likely, diagnostic testing should be performed (see below), and treatment with aciclovir should be started immediately, before the results of definitive investigations are available.(28) GRADE A Aciclovir should be considered for an unwell infant who is not showing clinical improvement and has negative bacterial cultures at 48–72 hours.(29) Please see the Treatment section below for more specific details of the treatment options for neonates with HSV infection.
Diagnosis
If vesicular lesions are present, the base of the lesion should be scraped and sent for PCR. Getting an adequate specimen for analysis requires operator expertise, and therefore a negative PCR result should be interpreted with caution.
Given that neonatal HSV infection can be present even in the absence of skin lesions, other diagnostic specimens are also required. Therefore, swabs should be taken from the nasopharynx/mouth, conjunctiva, umbilicus, rectum and urine. It is best to take swabs when the infant is >24 hours of age to avoid possible contamination from maternal cervico-vaginal secretions; positive results after 24 hours should reflect viral replication.
A CSF sample should be taken for HSV PCR testing, and evaluation of other parameters such as cell count, protein and glucose. Whole blood PCR should also be performed to assist with diagnosing neonatal HSV infection.
PCR is a rapid, highly sensitive and specific technique that detects tiny quantities of viral DNA. It is more reliable than viral culture for CNS infections. However, although a positive PCR result is highly predictive of infection, a negative result does not eliminate the possibility of disease.(30) A negative PCR finding from a CSF sample should be evaluated in conjunction with the overall clinical picture, including other diagnostic modalities, and should not be used on its own to exclude CNS HSV infection. GRADE A
Liver function tests, including serum transaminases, may indicate HSV hepatitis and a chest x-ray may reveal pneumonitis.(25, 31) A full blood count should be obtained to check for thrombocytopenia. It is recommended that these tests are performed on all infants suspected of having neonatal HSV infection. GRADE A
For CNS disease, neurological imaging (computed tomography [CT] or magnetic resonance imaging [MRI] scan of the brain) and an electroencephalogram should be considered as an important adjunct to diagnosis. MRI and CT scan findings may be normal early in the disease course and therefore do not categorically rule out HSV CNS disease.
An ophthalmology consultation should be sought in suspected or confirmed cases of neonatal HSV infection to help identify and monitor ocular complications that may arise during the illness. GRADE C
Finally, a sexual history must be taken from the parents, and maternal genital secretions should undergo HSV PCR and HSV serology. This is important, even when neonatal HSV infection presents weeks after delivery.
Treatment
Intravenous antivirals
Treatment with intravenous aciclovir decreases the mortality and morbidity associated with neonatal HSV infections (Table 1).(25, 28, 32)
- Aciclovir 20mg/kg every 8 hours for 14-21 days (depending on symptoms and CNS involvement).
Starting treatment early improves neurological outcomes. Aciclovir treatment should be continued for 14 days in neonates with SEM disease, and for at least 21 days in those with CNS and/or disseminated infections.(32) The longer duration of therapy is also recommended for infants with SEM disease who have abnormal CSF parameters, including HSV DNA detected by PCR. GRADE A & B
Supportive care includes: respiratory support, seizure management, fluid/electrolyte balance, correction of coagulopathy, and treatment of concurrent bacterial sepsis if present.
Maintain contact precautions while skin lesions are present and for at least 4 weeks in hospital.
Topical (ocular) antivirals
Neonates with ocular involvement may require topical therapy, and this should be managed by an ophthalmologist. Aciclovir treatment for 10 days is recommended for high-risk asymptomatic infants without laboratory confirmation of HSV infection.(33, 34)
At the end of the recommended duration of therapy with aciclovir, all infants with HSV CNS involvement should have a lumbar puncture to determine whether the CSF is PCR negative for HSV. If the CSF is still PCR positive for HSV, the American Academy of Pediatrics recommends repeating PCR of a CSF sample one week later, and again after another week if the second test was still positive; if the result is persistently positive then the case should be discussed with an infectious disease specialist.(35) Others have suggested that PCR positive neonates should continue to receive intravenous aciclovir until viral DNA is no longer detected in the CSF.(24, 25) GRADE B Aciclovir-resistant neonatal HSV infection is rare.
A double-blind, placebo-controlled study found that infants surviving neonatal HSV disease with CNS involvement had improved neurodevelopmental outcomes when they received suppressive therapy with oral aciclovir 300 mg/m2/dose given 3 times daily for 6 months (approximately 20 mg/kg, three times daily).(33) Suppressive antiviral therapy with oral aciclovir has been shown to reduce skin recurrences in infants with HSV infection, but regular neutrophil count monitoring is required because 20–25% of infants developed neutropenia during treatment with aciclovir.(36) Oral valaciclovir has not been evaluated for use as suppressive therapy. GRADE A
Suppressive therapy can be considered in infants with recurrent SEM disease, but it has not been shown to alter neurological outcomes.(33)
Suppressive oral aciclovir
Consider for infants with CNS or disseminated disease, and for those with recurrent SEM. Suppressive therapy reduces recurrence rates and improves neurodevelopmental outcomes in these conditions.
- Aciclovir 20mg/kg every 8 hours for 6 months
Monitoring: Full blood count should be checked regularly; ~20-25% of infants develop neutropenia during prolonged therapy.
General management points
Monocytic leukocytosis in the CSF could indicate CNS HSV infection.(37) Treatment with aciclovir should be instituted before HSV PCR results are available. This can be discontinued if an alternative diagnosis is established or the clinical course is no longer compatible with HSV CNS disease, viral PCR testing is negative, and/or scan results are normal or are not indicative of HSV encephalitis. However, it is important to be aware that a negative initial HSV PCR result does not exclude CNS disease. Neonatal HSV CNS infection may occur despite normal CSF counts and biochemistry, and the CSF HSV PCR result may be negative (especially if the lumbar puncture was performed early in the disease course).(25, 38) Therefore, repeat lumbar puncture is recommended when laboratory tests are negative but clinical suspicion remains high. GRADE B & C
Empirical treatment with aciclovir is recommended in the following situations: an infant remains critically ill despite antibiotic therapy and disseminated HSV cannot be excluded; if bacterial cultures are negative; or there are signs of progressive liver dysfunction with coagulopathy.(39) GRADE C
In addition to the administration of aciclovir, other important aspects of managing an infant with neonatal HSV infection include respiratory support, control of circulation, management of seizures, maintenance of fluid and electrolyte balance, correction of coagulopathy, and administration of antibiotics for concomitant bacterial infections.
Infants with neonatal HSV disease should be managed using contact isolation for 4 weeks if admitted or throughout the course of their illness.(40) This is inclusive of caregivers. GRADE C
The Australasian Society for Infectious Diseases (ASID) released flowcharts in 2022 advising on the management of a positive HSV diagnosis in neonates.
Follow-up
Long-term follow-up in survivors of neonatal HSV infection is important to monitor for longer-term sequelae. This includes assessment of hearing, vision and neurodevelopment. GRADE C
A full clinical examination should be performed when a cutaneous recurrence occurs. If there is any evidence of systemic involvement (e.g. fever, and especially irritability), a CSF examination, including HSV DNA PCR, should be performed. There should always be a low level of suspicion in the decision to initiate parenteral aciclovir therapy. If abnormal test results are then obtained, the course of intravenous aciclovir can be prolonged, then followed with suppressive oral aciclovir therapy until at least 6 months of age. GRADE C
Counselling for parents of an infant diagnosed with neonatal HSV infection
A diagnosis of neonatal HSV infection causes considerable stress for families, and many couples go on to separate.(41) Factors contributing to relationship breakdown include concern over a critically ill infant exacerbated by guilt over transmission of the virus, and the demands of long-term care for an often severely impaired child. Therefore, expert education and counselling is required. GRADE C
Being comfortable with discussing their infant’s diagnosis (what, why, how, etc.) is critical to parents’ ability to understand and come to terms with what has happened. Points specific to the neonatal HSV infection setting are provided below, but additional information can also be found in the separate Counselling a Diagnosis chapter.
- Parents are likely to be shocked, and feeling both grief and shame, which may be expressed as anger and/or withdrawal from staff.
- The diagnosis and having a sick infant could trigger or exacerbate relationship issues. It is helpful if health professionals take time to listen and do not attribute blame to either parent.
- Parents need to know that healthcare providers do not blame them for the baby contracting HSV (note that attitudes can be conveyed verbally and non-verbally).
- Although either parent may have known that they had HSV, it is quite common for people to have previously undiagnosed HSV infection.
- Most neonatal HSV infection occurs when a person experiences a ‘silent’ (asymptomatic) primary episode in late pregnancy.
- A primary episode of HSV-1-related genital herpes in late pregnancy is associated with a high risk of neonatal transmission.
- Many people do not realise that cold sores are caused by HSV and may be passed to the genital area during oral sex.
- Given the social stigma of sexually transmitted infections, parents may be unable to initiate a conversation with healthcare providers or ask the questions that are worrying them. Health professionals therefore need to take the initiative in addressing possible concerns. Starting with, “many parents wonder about… is this a concern for you?” is useful for normalising parental queries.
- Health professionals need to convey that they are comfortable talking about adult sexuality, that intercourse and oral sex are normal practices when a person is pregnant, and that HSV may have been transmitted during sexual activity in pregnancy.
- Health professionals may need to initiate a conversation about sexual transmission, e.g. “would it be helpful if I explained to you how the virus is passed on?”
- Parents should be advised about transmission precautions with the neonate in regards to the risk of transmission to other children or family members.
- Parents can also be referred to www.herpes.org.nz, and the Herpes Helpline (tollfree 0508 11 12 13 or 09 433 6526 from a mobile).
Anticipatory management of neonates with known risk for neonatal HSV(34, 42)
High risk
Infants at known high risk for neonatal HSV infection are those born to pregnant people who experience their first episode of genital herpes during late pregnancy. Neonates in this category should be examined by a paediatrician experienced in identifying the signs of neonatal HSV infection. GRADE C
Pregnant people with first episode genital HSV infection associated with either genital lesions or subclinical shedding at delivery have a 25–57% chance of transmitting HSV to their babies if they deliver by the vaginal route.(19) Although not completely protective against neonatal HSV disease, elective Caesarean section significantly reduces the risk of transmission and is recommended for pregnant people who have a known or presumed first episode of genital herpes within 6 weeks of delivery, even if receiving suppressive antiviral therapy.(19) GRADE B
Because of the high risk of infection, an asymptomatic infant inadvertently delivered vaginally from a person with active first episode genital lesions should be managed as having suspected neonatal HSV infection. This means the immediate collection of specimens, including CSF for cell count, chemistry and PCR testing, HSV blood PCR, full blood count, liver function tests, and surface HSV PCR swabs. These tests should ideally be performed at 24 hours after delivery but can be done earlier if clinically indicated. Anticipatory aciclovir therapy should be started. The required duration of aciclovir therapy will depend on surface HSV PCR and CSF results. The mother’s total and type-specific HSV serological status should also be checked to confirm that this is a first episode of genital herpes and not a recurrence. GRADE C
When a mother has active first episode genital lesions and is febrile, has ruptured membranes for more than 4 hours, or when foetal scalp electrodes or forceps have been used (irrespective of the mode of delivery), the infant should be managed as if they have suspected neonatal HSV infection. GRADE C
Anticipatory aciclovir therapy can be discontinued if the neonate remains well, HSV PCR and molecular diagnostic testing have not identified HSV, and the CSF studies (including PCR results) are normal. If only the HSV PCR of surface swabs is positive and the neonate remains clinically well then aciclovir treatment should be continued for 10 days.(42) Aciclovir should be continued for 14 days when HSV is identified but CSF results are normal, and for 21 days if there is an abnormal CSF finding.(43) GRADE B & C
Low risk
Infants in this category are those born to a mother with a first episode of genital herpes during early-mid pregnancy or a mother with recurrent genital lesions at the time of delivery. These neonates should be examined by a paediatrician experienced in identifying the signs of neonatal HSV infection. GRADE C
Anticipatory guidance including surveillance HSV PCR testing (see details below), but no empiric aciclovir, is appropriate infants who appear well, do not have skin or mucosal lesions at birth, and have a mother in one of the following categories: GRADE B & C
- First episode of genital herpes more than 6 weeks before delivery.
- First episode of genital herpes within 6 weeks of delivery of an infant by elective Caesarean section.
- Active recurrent genital herpes at delivery.
- History of recurrent genital herpes during this pregnancy.
Anticipatory guidance
- Document the risk of neonatal HSV infection on the infant’s chart.
- Notify the infant’s lead maternity caregiver and general practitioner of this risk.
- Educate parents on the specific risk of neonatal HSV infection in their case and instruct them to report signs of fever, respiratory distress, jaundice, lethargy or irritability, poor feeding, skin, and eye or oral mucosal lesions in their infant.
- If clinical symptoms, or skin, eye or mucosal lesions appear then the infant should be managed based on having suspected neonatal HSV infection.
Surveillance HSV PCR testing
- HSV PCR swabs should be taken at 24–48 hours of age (swabs should not be taken at birth or within the first 24 hours of life due to possible contamination by maternal cervico-vaginal secretions).
- HSV PCR swabs should be obtained from eyes (conjunctiva), mouth, nasopharynx, umbilicus, urine and rectum.
- Additional clinical and laboratory evaluation for suspected neonatal HSV infection is indicated followed immediately by aciclovir therapy if HSV PCR testing is positive.(28) GRADE A
For caregivers who develop lesions after delivery
Caregivers should be told about the importance of hand washing. If HSV lesions are present on the breast: avoid feeding from that breast, discard expressed milk, use strict hygiene. Suppressive antivirals may be used for recurrent breast lesions. Parents with recently acquired or reactivated oral or other skin lesions need to take extra care when handling their baby. As well as hand washing, caregivers should cover affected skin sites and, for herpes labialis or stomatitis, wear a surgical mask and not kiss the baby until the lesions have crusted, dried and healed.
References
- Braig S, Chanzy B. Management of genital herpes during pregnancy: the French experience. Herpes 2004; 11 (2): 45-7.
- Poeran J, Wildschut H, Gaytant M, et al. The incidence of neonatal herpes in The Netherlands. J Clin Virol 2008; 42 (4): 321-5.
- Malm G, Berg U, Forsgren M. Neonatal herpes simplex: clinical findings and outcome in relation to type of maternal infection. Acta Paediatr 1995; 84 (3): 256-60.
- Kropp RY, Wong T, Cormier L, et al. Neonatal herpes simplex virus infections in Canada: results of a 3-year national prospective study. Pediatrics 2006; 117 (6): 1955-62.
- Clarke E, Patel R, Dickins D et al. Joint British Association for Sexual Health and HIV and Royal College of Obstetricians and Gynaecologists national UK guideline for the management of herpes simplex virus (HSV) in pregnancy and the neonate (2024 update). International Journal of STD & AIDS 2024; 0 (0): 1-20.
- Flagg EW, Weinstock H. Incidence of neonatal herpes simplex virus infections in the United States, 2006. Pediatrics 2011; 127 (1): e1-8.
- Corey L, Wald A. Maternal and neonatal herpes simplex virus infections. N Engl J Med 2009; 361 (14): 1376-85.
- Jones CA, Raynes-Greenow C, Isaacs D. Population-based surveillance of neonatal herpes simplex virus infection in Australia, 1997-2011. Clin Infect Dis 2014; 59 (4): 525-31.
- Morris A, Ridley GF, Elliott EJ. Australian Paediatric Surveillance Unit : progress report. J Paediatr Child Health 2002; 38 (1): 8-15.
- Gardella C, Handsfield HH, Whitley R. Neonatal herpes - the forgotten perinatal infection. Sex Transm Dis 2008; 35 (1): 22-4.
- Woestenberg PJ, Tjhie JH, de Melker HE, et al. Herpes simplex virus type 1 and type 2 in the Netherlands: seroprevalence, risk factors and changes during a 12-year period. BMC Infect Dis 2016; 16: 364.
- Gardella C, Brown Z. Prevention of neonatal herpes. Bjog 2011; 118 (2): 187-92.
- Brown ZA, Wald A, Morrow RA, et al. Effect of serologic status and cesarean delivery on transmission rates of herpes simplex virus from mother to infant. JAMA 2003; 289 (2): 203-9.
- Ozouaki F, Ndjoyi-Mbiguino A, Legoff J, et al. Genital shedding of herpes simplex virus type 2 in childbearing-aged and pregnant women living in Gabon. Int J STD AIDS 2006; 17 (2): 124-7.
- Corey L, Wald A. Sexually transmitted diseases. 3rd ed: McGraw-Hill; 1999.
- Remington JS, Klein JO, Wilson CB, Baker CJ. Infectious diseases of the fetus and newborn infant. 6th ed. Amsterdam: Elsevier; 2006.
- Caviness AC, Demmler GJ, Selwyn BJ. Clinical and laboratory features of neonatal herpes simplex virus infection: a case-control study. Pediatr Infect Dis J 2008; 27 (5): 425-30.
- Kimberlin DW. Herpes simplex virus infections of the newborn. Semin Perinatol 2007; 31 (1): 19-25.
- Brown ZA, Selke S, Zeh J, et al. The acquisition of herpes simplex virus during pregnancy. N Engl J Med 1997; 337 (8): 509-15.
- Randolph AG, Washington AE, Prober CG. Cesarean delivery for women presenting with genital herpes lesions. Efficacy, risks, and costs. JAMA 1993; 270 (1): 77-82.
- Looker KJ, Garnett GP, Schmid GP. An estimate of the global prevalence and incidence of herpes simplex virus type 2 infection. Bull World Health Organ 2008; 86 (10): 805-12, a.
- Elder DE, Minutillo C, Pemberton PJ. Neonatal herpes simplex infection: keys to early diagnosis. J Paediatr Child Health 1995; 31 (4): 307-11.
- Whitley RJ. Neonatal herpes simplex virus infections. J Med Virol 1993; 41 (Suppl 1): 13-21.
- Kimberlin DW, Lin CY, Jacobs RF, et al. Natural history of neonatal herpes simplex virus infections in the acyclovir era. Pediatrics 2001; 108 (2): 223-9.
- Kimberlin DW, Lakeman FD, Arvin AM, et al. Application of the polymerase chain reaction to the diagnosis and management of neonatal herpes simplex virus disease. National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. J Infect Dis 1996; 174 (6): 1162-7.
- Whitley R, Arvin A, Prober C, et al. Predictors of morbidity and mortality in neonates with herpes simplex virus infections. The National Institute of Allergy and Infectious Diseases Collaborative Antiviral Study Group. N Engl J Med 1991; 324 (7): 450-4.
- Kimberlin D, Powell D, Gruber W, et al. Administration of oral acyclovir suppressive therapy after neonatal herpes simplex virus disease limited to the skin, eyes and mouth: results of a phase I/II trial. Pediatr Infect Dis J 1996; 15 (3): 247-54.
- Whitley R, Arvin A, Prober C, et al. A controlled trial comparing vidarabine with acyclovir in neonatal herpes simplex virus infection. Infectious Diseases Collaborative Antiviral Study Group. N Engl J Med 1991; 324 (7): 444-9.
- Caviness AC, Demmler GJ, Almendarez Y, Selwyn BJ. The prevalence of neonatal herpes simplex virus infection compared with serious bacterial illness in hospitalized neonates. J Pediatr 2008; 153 (2): 164-9.
- Kimberlin DW. Neonatal herpes simplex infection. Clin Microbiol Rev 2004; 17 (1): 1-13.
- Diamond C, Mohan K, Hobson A, et al. Viremia in neonatal herpes simplex virus infections. Pediatr Infect Dis J 1999; 18 (6): 487-9.
- Kimberlin DW, Lin CY, Jacobs RF, et al. Safety and efficacy of high-dose intravenous acyclovir in the management of neonatal herpes simplex virus infections. Pediatrics 2001; 108 (2): 230-8.
- Kimberlin DW, Whitley RJ, Wan W, et al. Oral acyclovir suppression and neurodevelopment after neonatal herpes. N Engl J Med 2011; 365 (14): 1284-92.
- Palasanthiran P, Starr M, Jones C. Management of perinatal infections: Australasian Society for Infectious Diseases; 2014.
- American Academy of Pediatrics. Herpes simplex. In: Kimberlin DW, Brady MT, Jackson MA, Long SS, eds. Red Book: 2015 report of the Committee on Infectious Diseases. Oak Grove, IL: American Academy of Pediatrics; 2015: 432-45.
- Gutierrez K, Arvin AM. Long term antiviral suppression after treatment for neonatal herpes infection. Pediatr Infect Dis J 2003; 22 (4): 371-2.
- Arvin AM, Whitley RJ, Gutierrez KM. Herpes simplex infections. In: Remington JS, Klein JO, Wilson CB, Baker CJ, eds. Infectious diseases of the fetus and newborn infant. Philadelphia: Elsevier Saunders; 2006: 845-66.
- De Tiège X, Héron B, Lebon P, et al. Limits of early diagnosis of herpes simplex encephalitis in children: a retrospective study of 38 cases. Clin Infect Dis 2003; 36 (10): 1335-9.
- Scott LL. Perinatal herpes: current status and obstetric management strategies. Pediatr Infect Dis J 1995; 14 (10): 827-32; discussion 32-5.
- Sakaoka H, Saheki Y, Uzuki K, et al. Two outbreaks of herpes simplex virus type 1 nosocomial infection among newborns. J Clin Microbiol 1986; 24 (1): 36-40.
- Kimberlin DW. Neonatal HSV infections: the global picture. Herpes 2004; 11 (2): 31-2.
- Kimberlin DW, Baley J. Guidance on management of asymptomatic neonates born to women with active genital herpes lesions. Pediatrics 2013; 131 (2): e635-46.
- Jones CA. Vertical transmission of genital herpes: prevention and treatment options. Drugs 2009; 69 (4): 421-34.


